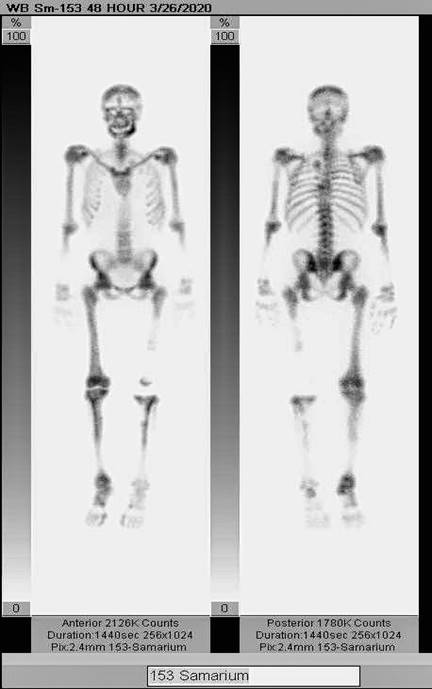
Sm-153 DOTMP is a clinical-stage bone targeting radiopharmaceutical originally developed by IsoTherapeutics Group LLC and now owned by IGL Pharma Inc.
Sm-153 DOTMP is out-licensed to QSAM Biosciences (QSAM), a company that Telix has signed an agreement to acquire. Telix’s acquisition of QSAM remains subject to customary closing conditions including regulatory approvals.
©2024 Telix Pharmaceuticals Limited. The Telix Pharmaceuticals® and IsoTherapeutics™ names and logos are trademarks of Telix Pharmaceuticals Limited and its affiliates – all rights reserved. This website is intended for corporate communication purposes only and is not intended as promotion or advertising to any audience in any country worldwide.

Melbourne (Australia) – 9 April 2024. Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces the completion of the acquisition of IsoTherapeutics Group, LLC (IsoTherapeutics).
IsoTherapeutics is a privately held, commercial-stage company that provides radiochemistry and bioconjugation development and contract manufacturing services to numerous companies in the radiopharmaceutical industry, including Telix. The acquisition further enhances Telix’s in-house development capabilities and expands Telix’s United States (U.S.) manufacturing footprint with particular focus on bioconjugation and isotope processing.
Dr Christian Behrenbruch, Managing Director and Group CEO of Telix said, “The acquisition of IsoTherapeutics Group is a significant milestone in Telix’s continued focus on vertical integration of development, supply and manufacturing and is highly complementary to Optimal Tracers (Sacramento, California), ARTMS (Vancouver) and our extensive commercial manufacturing infrastructure in Belgium. In the IsoTherapeutics team, we have partnered with some of the leading experts in radiochemistry and I am excited at what we can achieve together going forward.”